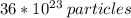Answer:
A
Explanation:
1)The mole is a unit of measure that contains as many elementary entities as in atoms of 12 grams of Carbon (12 AMU). The Carbon mass is the reference.
2)We have to specify which particle we are using when dealing with mole unit:
particle, atoms, molecules, ions, electrons, etc.
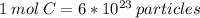
3) So If I have 6 moles of a substance, I am going to have six times the number of particles that are in 12 grams of carbon-12.