Answer:
 is the correct choice that is option A.
is the correct choice that is option A.
Step-by-step explanation:
An electron has
 times the mass of a proton, but an equal and opposite negative charge.
times the mass of a proton, but an equal and opposite negative charge.
Lets
 is any element represented here.
is any element represented here.
Where
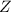 is the number of protons as well as its atomic number.
is the number of protons as well as its atomic number.
And
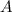 is the mass number which is the number of protons and neutrons in any atom.
is the mass number which is the number of protons and neutrons in any atom.
Note: We know that Hydrogen
 is devoid of neutron,so number of neutrons in a Hydrogen atom is zero.
is devoid of neutron,so number of neutrons in a Hydrogen atom is zero.
Then
 is also a representation of single proton.
is also a representation of single proton.
So
 is the right choice that is option A.
is the right choice that is option A.