Answer:
(a) The rate at which ammonia being formed = 0.0493 M/s
(b) The rate at which molecular nitrogen reacting = 0.0247 M/s
Step-by-step explanation:
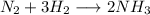
The rate at which molecular hydrogen is reacting =
![(d)/(dt)[H_(2)]=0.074\:M/s](https://img.qammunity.org/2020/formulas/chemistry/middle-school/2k683asezrxpz2i2z0bxkk2fedbt6ivhvx.png)
The rate of the above reaction can be expressed as:
![rate=-(d)/(dt)[N_(2)]=-(1)/(3) (d)/(dt)[H_(2)]=(1)/(2)(d)/(dt)[NH_(3)]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/qsnigm9x8zsdyyh5xve5a9seq3mpzom49s.png)
(a)
![(d)/(dt)[NH_(3)]=(2)/(3)(0.074)=0.0493](https://img.qammunity.org/2020/formulas/chemistry/middle-school/brgpyup4itz0bmh3yt1697zmkubzio9a6c.png)
The rate at which ammonia being formed = 0.0493 M/s
(b)
![-(d)/(dt)[N_(2)]=(1)/(3)(0.074)=0.0247](https://img.qammunity.org/2020/formulas/chemistry/middle-school/i9lszrzh7u6zc7pujnh93fzmuu6wbxhv22.png)
The rate at which molecular nitrogen reacting = 0.0247 M/s