Answer:
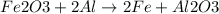
Step-by-step explanation:
Hello!
In this case, when balancing chemical reactions, we must make sure that the atoms of each element are the same at both reactants and products; thus, for the given reaction, we need two iron and aluminum atoms at each side based on their subscripts in the given oxides as shown below:
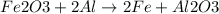
Best regards!