Answer:
Option A that is
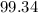 is the correct choice.
is the correct choice.
Explanation:
To find what percentage of carbon-14 is still remaining after
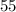 years.
years.
We have to pull the equation and instead of
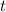 we will put the years in numbers that is
we will put the years in numbers that is
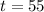
Lets see the equation.
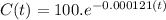
Now to find the carbon-14 percentage.
Putting the value of
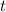 in years.
in years.
So
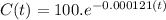 and
and
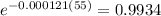
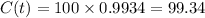
As mentioned that the function is already framed to find the percentage we need not to convert it or multiply with
 .
.
So the percentage of C-14 remaining after
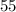 years is
years is
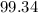
Option A is the correct choice.