Answer:
The pOH of the solution is 13.176
Step-by-step explanation:
The concentration of
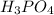 solution is 0.05 M
solution is 0.05 M
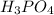 produces
produces
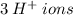 .
.
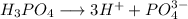
If the concentration of
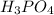 is 0.05 M, then the concentration of
is 0.05 M, then the concentration of
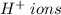 is three times that of
is three times that of
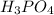 .
.
![[H^(+)]=3*0.05=0.15M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/tmsfdeea96vy64yof74dqy7vxrawq5g27l.png)
pH =-㏒(
![[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/axnvi9gez4h3rovfp1qidd16ya8d7gzbon.png) )= -㏒(0.15) =0.824
)= -㏒(0.15) =0.824
pOH = 14 - pH = 13.176