Answer:
1121.08 millilitres of 0.223 M
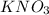 solution contains 0.250 moles of
solution contains 0.250 moles of
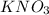 .
.
Step-by-step explanation:
The formula for molarity of a solution:
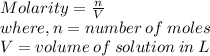
Molarity = 0.223 M
n = 0.250 moles
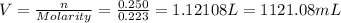
Therefore, 1121.08 millilitres of 0.223 M
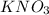 solution contains 0.250 moles of
solution contains 0.250 moles of
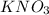 .
.