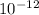The wavelength is 91.5 pm ( 91.5 Pico meter).
Explanation:
The formula can be expressed below for electron’s energy,
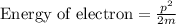
Where,
p = momentum
m= mass of electron
We know, mass of electron =
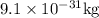
Energy of electron,
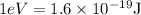
Therefore,
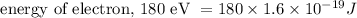
By substituting the known values in the equation, we get,
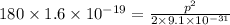
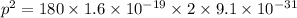
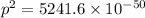
Taking square root, we get
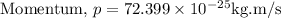
We know,
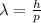
Here, h – Planck constant =
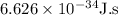
So, the wavelength would be,
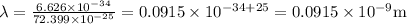
Adding
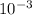 in both numerator and denominator we get the value as
in both numerator and denominator we get the value as
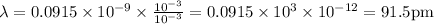
Where, pm – Pico meter -