Answer:
Hi, the given equation has some missing parts. Actual equation is- '
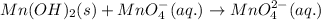 '
'
balanced equation:
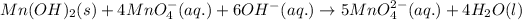
Step-by-step explanation:
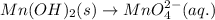
Balance O and H in basic medium:
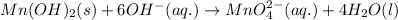
Balance charge:
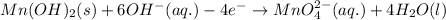 ........(1)
........(1)
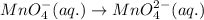
Balance charge:
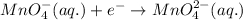 .....(2)
.....(2)
![[equation(2)* 4]+[equation (1)]:](https://img.qammunity.org/2020/formulas/chemistry/high-school/s7zv0ex3mikso5cx14qy4ag7eha0w75qyb.png)
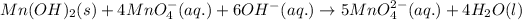
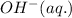 is present on the left hand side of balanced equation and it's coefficient is 6
is present on the left hand side of balanced equation and it's coefficient is 6