Answer:
-41,9kJ/mol NaOH
Step-by-step explanation:
For the solution process:
NaOH(s) →Na⁺(aq) + OH⁻(aq)
The released heat is:
Q = -C×m×ΔT
Where Q is the released heat, C is specific heat of the solution (4,18J/g°C), m is the mass of water (100,0g) and ΔT is (32,0°C-23,9°C)
Replacing:
Q = -3385,8J
This heat is released per 3,23g of NaOH. Now, the heat released (ΔH) per mole of NaOH is:
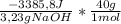 = -41929J/molNaOH ≡
= -41929J/molNaOH ≡
-41,9kJ/mol NaOH
I hope it helps!