Answer:
C) P3– > Cl– > K+ > Ca2+
Step-by-step explanation:
Ionic radius can be defined as the distance between the nucleus and the electron in the outermost shell of atom in its ionic state. When an atom looses an electrons(Cation) its ionic radius decreases, whereas when an atom gains an electron(anion), its ionic radius increases.
Among the given ions the ionic radius of
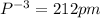
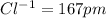
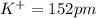
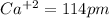
all distances are in picometer.
clearly,
option C is correct that is
C) P3– > Cl– > K+ > Ca2+