Answer:
0.639 V
Step-by-step explanation:
The volatge of the cell containing both Ag/AgCl reference electrode and
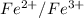 electrode = 0.693 V
electrode = 0.693 V
Thus,

E_{anode}=0.197 V
Note: potential of the silver-silver chloride reference electrode (0.197 V)
⇒E_{Cthode}= 0.693+0.197 = 0.890V
To calculate the voltage of the cell containing both the calomel reference
electrode and
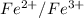 electrode as follows
electrode as follows
Voltage of the cell =

E_{anode}= calomel electrode= 0.241 V
Voltage of the cell = 0.890-0.241 = 0.639 V
Therefore, the new volatge is = 0.639 V