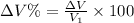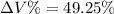Answer:
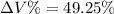
Step-by-step explanation:
Assuming the pressure inside the packet at the time of packaging,
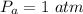
Given that there is no temperature difference between the air inside the pack and the cabin.
Using mathematical expression of Boyle's law:
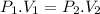
where subscripts 1 & 2 denote the initial and final conditions respectively.
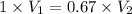
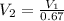
Now change in volume:
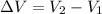
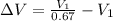
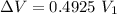
Now the % increase in volume: