Answer:
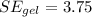
Step-by-step explanation:
First, we have to calculate the gel's column height using the cylinder's volume, as follows:
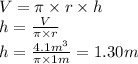
Then, as the pressure given at the bottom of the tank is the sum of the surface pressure and the gel's column pressure, we need to calculate only the gel's column pressure:
ft of water is a unit of pressure, but we need to convert it to atm and then to Pa, in order to calculate our results in the correct units. Therefore, the conversion factor is:
1 ft of water (4°C) = 0.0295 atm
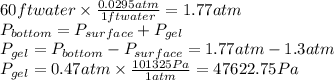
Now, to calculate the specific gravity, we need to find first the gel's density:
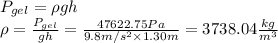
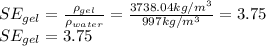
The specific gravity of the gel is 3.75.