Answer:
11.0L of carbon dioxide is produced
Step-by-step explanation:
Balanced equation:
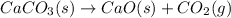
According to balanced equation, 1 mol of
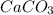 produces 1 mol of
produces 1 mol of

So, 0.489 mol of
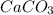 produces 0.489 mol of
produces 0.489 mol of

Let's assume
 behaves ideally.
behaves ideally.
So,
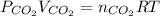
where P is pressure, V is volume , n is number of moles, R is gas constant and T is temperature in kelvin
Plug-in all the values in the above equation-
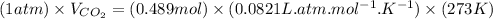
or,
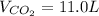
So, 11.0L of carbon dioxide is produced