Answer:
1. 0.0637 moles of nitrogen.
2. The partial pressure of oxygen is 0.21 atm.
Step-by-step explanation:
1. If we assume ideal behaviour, we can use the Law of ideal gases to find the moles of nitrogen, considering that air composition is mainly nitrogen (78%), oxygen (21%) and argon (1%):
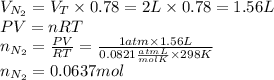
2. Now, in order to find he partial pressure of oxygen we need to find the total moles of air, and then the moles of oxygen. Then, we use these results to determine the molar fraction of oxygen, to multiply it with total pressure and get the partial pressure of oxygen as follows:
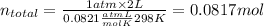
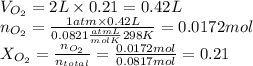
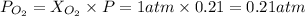
As you see, the molar fraction and volume fraction are the same because of the assumption of ideal behaviour.