Answer:
The volume (in ml) of a 3.57 M solution of
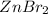 that contains 0.884 moles of
that contains 0.884 moles of
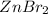 is 247.61g mL
is 247.61g mL
Step-by-step explanation:
According to the definition of Molarity
which sates
"The amount of moles of a substance present in one litre of the solution is called as Molarity."
3.57M
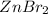 solution implies that 1000 mL of the solution conatins 3.57Moles of
solution implies that 1000 mL of the solution conatins 3.57Moles of
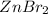
Hence , 3.57 moles of
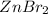 is present in the 1000 ml of solution
is present in the 1000 ml of solution
Now,
In 1 mole of
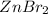 is present in the =
is present in the =
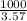 ml of solution
ml of solution
Similarily ,
In 0.884 moles of
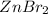 is present in the
is present in the
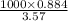 of solution= 247.61g mL
of solution= 247.61g mL