Answer:
4.99 mg of vitamin C are in the beaker.
Step-by-step explanation:
Given that,
Weight of vitamin = 0.0499 g
Molar mass = 176.124 g/mol
Weight of water = 100.0 ml
We need to calculate the mg of vitamin C in the beaker
We dissolve 0.0499 g vitamin C in water to from 100.0 ml solution.
100 ml solution contain 49.9 mg vitamin C
Now, we take 10 ml of this vitamin C solution in breaker
Since, 100 ml solution =49.9 mg vitamin C
Therefore,
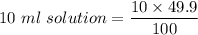
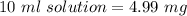
Hence, 4.99 mg of vitamin C are in the beaker.