Answer:
1 billion molecules O₂
Step-by-step explanation:
From my research, a human red blood cell contains approximately 270 million hemoglobin molecules.
A hemoglobin molecule contains four heme groups, each of which has an iron ion forming a coordination complex that carries every dioxygen molecule. Therefore for each hemoglobin molecule, we will have 4 dioxygen molecules. The heme groups are responsible for the transport of every dioxygen and other diatomic gases.
Hence, the number of O₂ molecules in a red blood cell saturated with 100% will be:
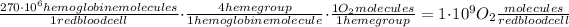
So, the correct answer is 1 billion of O₂ molecules.
Have a nice day!