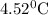Answer:
Change in temperature of calorimeter is
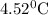
Step-by-step explanation:
Molar mass of anethole = 148.2 g/mol
So, 0.950 g of anethole =
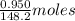 of anethole = 0.00641 moles of anethole
of anethole = 0.00641 moles of anethole
1 mol of anethole releases 5541 kJ of heat upon combustion
So, 0.00641 moles of anethole release
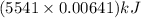 of heat or 35.52 kJ of heat
of heat or 35.52 kJ of heat
7.854 kJ of heat increases
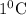 temperature of calorimeter.
temperature of calorimeter.
So, 35.52 kJ of heat increases
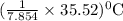 or
or
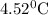 temperature of calorimeter
temperature of calorimeter
So, change in temperature of calorimeter is