Answer:
a)
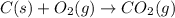
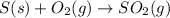
b)
Sulphurdioxide
c)
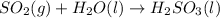
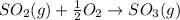
d)
Incomplete combustion produce harmful gases.
Step-by-step explanation:
a)
Carbon reacts with atmospheric oxygen to form carbondioxide as well as sulphur dioxide.
The chemical equations are as follows.
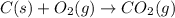
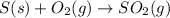
b)
Sulfur dioxide is very harmful to the environment to cause acid rains.
This harmful gas mix with rain water to form sulphuric acid.
c)
The balanced chemical equation for the reaction that produces harmful environmental effects is as follows.
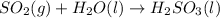
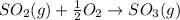
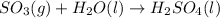
d)
In the absence of proper amount of oxygen required for combustion, incomplete combustion will take place which will result in formation of more carbondioxide an other harmful gases.