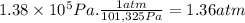Answer:
1.36 atm
Step-by-step explanation:
First, we have to transform lb and in² into the International System of Units.
- The international unit for force is the Newton. The equivalence (based on Earth's gravity) is 1 lb = 4.448 N.
- The international unit for area is the m². The equivalence is 1 m² = 1550 in².
- The resulting unit is known as Pascal. 1 Pa = 1 N/m².
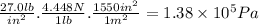
The equivalence between Pascal and atm is 101,325 Pa = 1 atm. Then,