Answer:



Step-by-step explanation:
c = Speed of light =
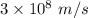
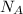 = Avogadro's number =
= Avogadro's number =

 = Frequency
= Frequency
 = Wavelength
= Wavelength
The minimum energy is given by

The minimum energy is

The energy of a photon is given by

The frequency of the photon is

Wavelength is given by

The wavelength is
