Answer:
60.0 years must pass to reduce a 24 mg of cesium 237 to 6.0 mg
Step-by-step explanation:
For radioactive decay of a radioactive nuclide-
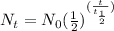
Where,
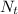 is amount of radioactive nuclide after "t" time , N_{0} is initial amount of radioactive nuclide and
is amount of radioactive nuclide after "t" time , N_{0} is initial amount of radioactive nuclide and
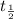 is half-life of radioactive nuclide
is half-life of radioactive nuclide
Here N_{0} = 24 mg, N_{t} = 6.0 mg and
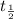 = 30.0 years
= 30.0 years
So,
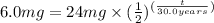
or,
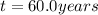
So 60.0 years must pass to reduce a 24 mg of cesium 237 to 6.0 mg