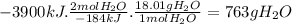Answer:
763 g
Step-by-step explanation:
Let's consider the following thermochemical equation.
SiO₂(s) + 4 HF(g) → SiF₄(g) + 2 H₂O(l) ΔH°rxn = -184 kJ
From the enthalpy of the reaction (ΔH°rxn), we can affirm that 184 kJ are released when 2 moles of H₂O(l) are produced. Taking into account that the molar mass of H₂O is 18.01 g/mol, the mass of water formed to produce 3900 kJ of energy is: