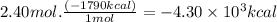Answer:
-4.30 × 10³ kcal
Step-by-step explanation:
Let's consider the following thermochemical equation for the combustion of acetone.
C₃H₆O(l) + 4 O₂ (g) → 3 CO₂(g) + 3 H₂O(g) ΔH°of the reaction = -1790 kcal
When 1 mole of C₃H₆O burns, 1790 kcal of heat are released. We have to find out how many moles of C₃H₆O reacted in 177 mL. Considering the density of acetone is 0.788 g/mL and its molar mass is 58.08 g/mol, the moles of acetone are:
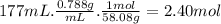
The heat released when 2.40 moles of acetone burn is: