Answer:
Three times
Step-by-step explanation:
The amount of heat added to a substance when its temperature is increased from T_1 to T_2 is given by
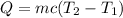
c= specific heat capacity
m= mass of the substance
⇒Q∝c
that is if c is increased three times the amount of heat added is also increased three times. Therefore, the amount of heat added to the copper is three times the heat added to lead.