Answer:
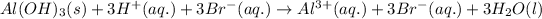
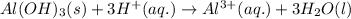
Step-by-step explanation:
Aluminium hydroxide (
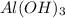 ) is a base and hydrobromic acid (HBr) is a strong acid.
) is a base and hydrobromic acid (HBr) is a strong acid.
Hence an acid-base reaction occurs between
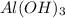 and HBr
and HBr
Balanced molecular equation:
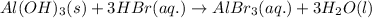
Balanced total ionic equation:
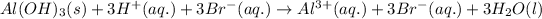
Balanced net ionic equation:
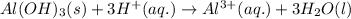
(net ionic equation is written by removing common ions present in both side of total ionic equation)