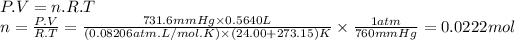Answer:
0.0222 mol
Step-by-step explanation:
When a gas is collected by water displacement, the total pressure is equal to the pressure of the gas (in this case oxygen) and the pressure of the water vapor.
P = Pw + PO₂
PO₂ = P - Pw = 754.0 mmHg - 22.38 mmHg = 731.6 mmHg
We can find the moles of oxygen using the ideal gas equation.