Answer:
The maximum mass of carbon dioxide that could be produced by the chemical reaction is 5.96 grams
Step-by-step explanation:
The balanced reaction between gaseous butane and oxygen occurs as follows:
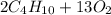 ⇒
⇒
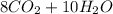
In order for the equation to be balanced, it was taken into account that the law of conservation of matter states that no atom can be created or destroyed in a chemical reaction, so the number of atoms that are present in the reagents has to be equal to the number of atoms present in the products.
Knowing the reaction that occurs between both reagents, it is possible to know the stoichiometry of the reaction (that is, the quantities of reagents necessary for a certain amount of products to be produced). And assuming that 3.49 g of butane are mixed with 7.0 g of oxygen it is possible to determine the limiting reagent, that is to say the reagent that is consumed first, by determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
In order to determine the limiting reagent, you must first determine the reacting mass of each reagent. Then you must first know the molar mass of butane and oxygen, taking into account the atomic mass of each element that composes it and the amount present:
Atomic masses:
- C: 12 g/mol
- H: 1 g/mol
- O: 16 g/mol
Molecular Mass:
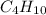 : 12 g/mol *4 + 1 g/mol *10= 58 g/mol
: 12 g/mol *4 + 1 g/mol *10= 58 g/mol- O₂: 16 g/mol *2= 32 g/mol
- CO₂: 12 g/mol + 16 g/mol *2= 44 g/mol
- H₂O: 1 g/mol *2 + 16 g/mol= 18 g/mol
Therefore, observing the reaction, 2 moles of butane and 13 moles of oxygen react. With the previously calculated molar masses it is possible to determine the mass that reacts by stoichiometry of each reagent.
Reactive mass of each reagent:
- C₄H₁₀= 2 moles* 58 g/mol= 116 g
- O₂= 13 moles* 32 g/mol= 416 g
Assuming that 3.49 g of butane react, and taking into account stoichiometry, it is possible to make a rule of three to determine the limiting reagent: if for 116 grams of butane to react, 416 grams of oxygen are needed, how many moles of oxygen are needed to 3.49 grams react of butane?
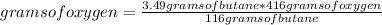
grams of oxygen= 12.52
Then the limiting reagent will be oxygen because a smaller amount of reagent (7 grams) is available. Then the following calculations are made from the available 7 grams of oxygen.
First, the amount of product that is produced by stoichiometry is determined, as was previously done with the reagents:
- CO₂: 8 moles* 44 g/mol= 354 g
- H₂O: 10 moles* 18 g/mol= 180 g
To determine the maximum mass of carbon dioxide that could cause the chemical reaction, a rule of three is applied taking into account the limiting reagent and stoichiometry: if by stoichiometry 416 grams of oxygen produce 354 grams of carbon dioxide, how many grams of product produce 7 grams of oxygen?
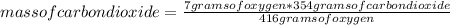
mass of carbon dioxide= 5.96 grams
Finally, the maximum mass of carbon dioxide that could be produced by the chemical reaction is 5.96 grams