Answer:
Step-by-step explanation:
The balanced chemical reaction is:
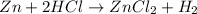
According to Dalton's law, the total pressure is the sum of individual pressures.
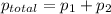
As the hydrogen is collected over water, the total pressure will be sum of pressure of water and pressure of dry hydrogen.
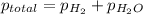
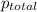 = 742.5 mm Hg
= 742.5 mm Hg
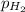 = ?
= ?
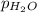 = 23.8 mm Hg
= 23.8 mm Hg
Putting in the values:
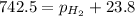
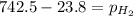
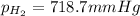
Thus the pressure of dry hydrogen gas is 718.7 mm Hg