Step-by-step explanation:
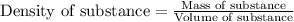
We are given:
Mass of liquid = 23.46 g
Volume occupied by the ring =
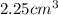
Density of gold ring = D
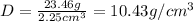
Original density of gold =
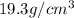
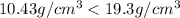
We will tell the buyer that gold ring purchased from him is too adulterated with other metals which have reduced its density indication that it is an artificial gold ring.