Answer:
Light of a shorter wavelength should be used.
Step-by-step explanation:
This is studied in the phenomenon called photoelectric effect, in which light is able to release electrons from a metal, said electrons are called photoelectrons .
The experiments that have been carried out show that increasing or decreasing the intensity of the light will not cause the photoelectrons to be emitted, what will cause the photoelectrons to be emitted is to increase the frequency of the incident light.
And a higher frequency corresponds to a shorter wavelength according to the equation:
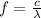
(where
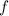 is frequency,
is frequency,
 the speed of light, and
the speed of light, and
 the wavelength)
the wavelength)
So the answer is that the wavelength of the light must be shortened to cause the emission of electrones.