Answer:
1.
The balanced chemical reaction
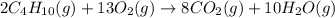
2.
The produced volume of carbondioxide is 596.6 L.
Step-by-step explanation:
1.
The combustion of butane in the presence of atmospheric oxygen to form carbondioxide.
The balanced chemical reaction is as follows.
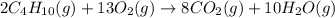
2.
from the given,
Mass of butane = 0.360 kg = 360 gm
Let's convert the kg into moles
Molar mass of butane = 58.12 g/mol
From the reaction 2 moles of butane gives 8 moles of carbon dioxide.
Hence,
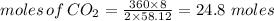
Let's calculate the volume of carbondioxide:

Rearrange the formula is as follows.
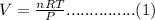
n= Number of moles = 24.8 moles
R = Gas constant = 0.0821 atm.L/mol.K
T = Temperature = 20 c = 20+273 = 293 K
P = Pressure = 1 atm
Substitute the all values in equation (1) then we get volume of carbondioxide.
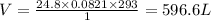
Therefore, The produced volume of carbondioxide is 596.6 L.