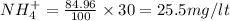Answer:
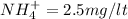
Step-by-step explanation:
Given data:
Ammonia Nitrogen 30 mg/L
pH = 8.5
-log[H +] = 8.5
[H +] = 10^{-8.5}
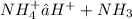
Rate constant is given as
![K_a = ([H^(+)] [NH_3])/(NH_4^(+))](https://img.qammunity.org/2020/formulas/engineering/college/xunux5yi5l31q8j7m8b7c6kbgt2uq9vaqs.png) ...........1
...........1
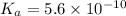
Total ammonia as NItrogen is given as 30 mg/l
![\%NH_4^(+) = ( [NH_4] * 100)/([NH_4^(+)] + [NH_3])](https://img.qammunity.org/2020/formulas/engineering/college/epb0yrihou7cnkr8id8af9wqjckwmryf42.png)
=
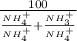
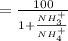 .....2
.....2
from equation 1 we have
![(NH_3^+)/(NH_4^+) =(K_a)/([H^+]) = \frac{5.6* 10^(-10)}](https://img.qammunity.org/2020/formulas/engineering/college/4cmcm1d6n9l1s6gvbhwix93biivptcs5ki.png) {10^{8.5}}
{10^{8.5}}
plug this value in equation 2 we get
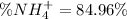
Total ammonia as N = 30 mg/lt