Step-by-step explanation:
Charles's law gives the relation between volume and the temperature of ideal gas when pressure is held fixed. It states that the volume of an ideal gas is directly proportional to its temperature at constant pressure.
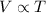
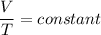
If the temperature of an ideal gas is doubled, its volume also gets doubled as there is a direct relation between volume and temperature.