Answer:
A. Add 30 J by heating the system and have the system do 20 J of work.
Step-by-step explanation:
The equation for internal energy is
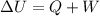 , where here Q is the heat added to the system (negative if removed from the system) and W is the work done on the system (negative if done by the system). From this is clear that if we add 30J of heat to the system and have it do 20J of work we will have
, where here Q is the heat added to the system (negative if removed from the system) and W is the work done on the system (negative if done by the system). From this is clear that if we add 30J of heat to the system and have it do 20J of work we will have
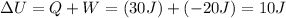 .
.