Answer :
(a) The value of
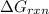 is -51.4 kJ/mol
is -51.4 kJ/mol
(b) Yes, the hydrolysis of ATP spontaneous under these conditions.
Explanation :
First we have to calculate the reaction quotient.
Reaction quotient (Q) : It is defined as the measurement of the relative amounts of products and reactants present during a reaction at a particular time.
The given balanced chemical reaction is,
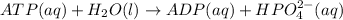
The expression for reaction quotient will be :
![Q=([ADP][HPO_4^(2-)])/([ATP])](https://img.qammunity.org/2020/formulas/chemistry/college/c9y995ymahj8pm8bqwstguejivdqczd0m3.png)
In this expression, only gaseous or aqueous states are includes and pure liquid or solid states are omitted.
Given:
![[ATP]](https://img.qammunity.org/2020/formulas/chemistry/college/yhnd218o8j6m4s62m5569d8fwtiu5nn0q3.png) = 5.0 mM
= 5.0 mM
![[ADP]](https://img.qammunity.org/2020/formulas/chemistry/college/77qtknfbboa4m4n9ek1ljnag1rxvreec3a.png) = 0.30 mM
= 0.30 mM
![[HPO_4^(2-)]](https://img.qammunity.org/2020/formulas/chemistry/college/t7c4zjgucnoa05itpdcogyuk923t6fwx6n.png) = 5.0 mM
= 5.0 mM
Now put all the given values in this expression, we get
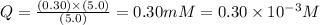
Now we have to calculate the value of
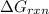 .
.
The formula used for
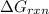 is:
is:
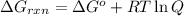 ............(1)
............(1)
where,
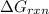 = Gibbs free energy for the reaction = ?
= Gibbs free energy for the reaction = ?
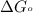 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol
R = gas constant =
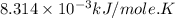
T = temperature =
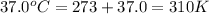
Q = reaction quotient =
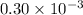
Now put all the given values in the above formula 1, we get:
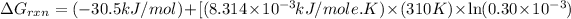
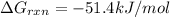
Therefore, the value of
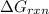 is -51.4 kJ/mol
is -51.4 kJ/mol
Gibbs free energy : It is defined as the amount of energy that is available to do useful work.
A reaction to be spontaneous when
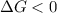
A reaction to be non-spontaneous when
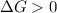
As the value of
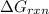 is less than 0. So, the reaction will be spontaneous.
is less than 0. So, the reaction will be spontaneous.
Yes, the hydrolysis of ATP spontaneous under these conditions.