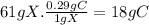Answer:
18g C
Step-by-step explanation:
A sample of chemical X is found to contain 5.0 grams of oxygen, 10.0 grams of carbon, and 20.0 grams of nitrogen. The mass of that sample is:
5.0 g + 10.0 g + 20.0 g = 35.0 g
The ratio mC/mX is:
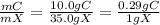
According to the law of definite proportion, this ratio is constant for the same chemical. For a sample of 61 g of X,