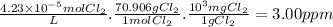Answer:
3.00 ppm
Step-by-step explanation:
The molar concentration of Cl₂ is 4.23 × 10⁻⁵ M = 4.23 × 10⁻⁵mol Cl₂/L. We want to convert this concentration to parts per million 1 ppm = 1mg/L.
We will use the following relations:
- The molar mass of Cl₂ is 70.906 g/mol.
- 1 g = 10³ mg
Then, we can use proportions: