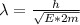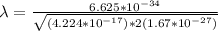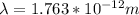To develop this problem it is necessary to apply the concepts related to electromagnetic energy and Broglie's hypothesis.
By definition we know that the electrical energy of a proton can be expressed as
E = qV
Where,
q = Charge of proton
V = Voltage
Replacing with our values
E = qV
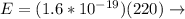 It is necessary to add the two potentials
It is necessary to add the two potentials
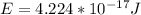
From Broglie's hypothesis we know that the wavelength is given by
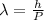
Where,
h = Planck's constant
p = Momentum
The momentum of a particle can be expressed in terms of energy, that is,
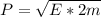
Where,
m = mass
E = Energy (potential or kinetic)
Therefore replacing this value at lambda,