Answer:
The tank with O₂ weighs more.
Step-by-step explanation:
We can find the mass of gas using the ideal gas equation.
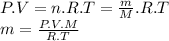
Considering the pressure (P), volume (V), temperature (T) and ideal gas constant (R) are the same, we can establish that:
m ∝ M
The mass is directly proportional to the molar mass. The molar mass of O₂ (32 g/mol) is higher than the molar mass of N₂ (28 g/mol). Therefore, the tank with O₂ weighs more.