Answer: equilibrium conditions will shift in left if the temperature in the lab is decreased.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
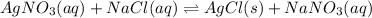
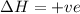
This is a type of Endothermic reaction because heat is absorbed in the reaction.
On decreasing the temperature :
If the temperature is decreased, so according to the Le-Chatelier's principle , the equilibrium will shift in the direction where increase in temperature occurs. As, this is an endothermic reaction, forward reaction will decrease the temperature. Hence, the equilibrium will shift in the left direction where temperature is increasing as backward reaction will be exothermic.
Thus the equilibrium conditions will shift in left if the temperature in the lab is decreased.