Answer:
C) specific heat capacity
Step-by-step explanation:
As we know that heat given to the system to change the temperature is given as
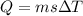
here we know that
Q = thermal energy given to the system
s = specific heat capacity of the system
 = change in temperature
= change in temperature
so here we know that
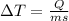
here we know that heat given to the two cubes is of same amount as well as the mass is same but the final temperature is different because of the specific heat capacity (s)
So correct answer would be
C) specific heat capacity