The given statement"If the pressure of a gas sample is quadrupled and the absolute temperature is doubled" is false.
Answer: Option B
Step-by-step explanation:
As we know the direct relationship between Pressure and Temperature by the Gay-Lussac’s Law,
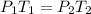
From this, we get,
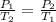
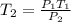
So, according to given statement, we have

Then from the above expression, we can find out the value of
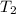 when pressure increased by 4 times of initial pressure as,
when pressure increased by 4 times of initial pressure as,
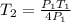
Hence, we get,

Hence, from the above expression we can say that as we increase the pressure four times, the temperature does not get doubled. So, the given statement in the question is false.