Answer:
The Structure of "B" is alkene.
Step-by-step explanation:
The compound "A" having R- configuration and undergoes Hofmann elimination to form an alkene.
The compound "B" on oxidatively cleaving with ozone followed by dimethyl sulfide forms
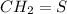 and
and
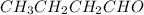
The structure of "A" and"B" is as follows.