Answer:
Theoretical yield of the reaction is 121·38 g
The excess reactant is hydrogen
The limiting reactant is nitrogen
Step-by-step explanation:
By assuming that the reaction between nitrogen and hydrogen taking place in presence of catalyst because at normal conditions the reaction between them will not occur
Number of moles of nitrogen taken are 100÷28 ≈ 3.57
Number of moles of hydrogen taken are 100÷2 = 50
Actually the reaction between nitrogen and hydrogen takes place according to the following equation
N
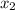 + 3H
+ 3H
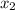 → 2NH
→ 2NH
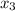
So from the equation for 1 mole of nitrogen and 3 moles of hydrogen we get 2 moles of ammonia
Here in the problem we have approximately 3·57 moles of nitrogen so we require 3×3·57 moles of hydrogen
∴ Number of moles of hydrogen required is 10·71
But we have 50 moles of hydrogen
∴ Excess reagent is hydrogen and limiting reagent is nitrogen
Number of moles of ammonia produced is 2×3·57 = 7·14
Weight of ammonia is 17 g
∴ Amount of ammonia produced is 17×7·14 = 121·38 g
∴ Theoretical yield of the reaction is 121·38 g