To solve this problem it is necessary to apply the concepts of heat change and thermal efficiency.
The heat rate can be expressed under the function
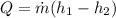
Where,
m = Mass
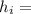 Enthalpy at each state
Enthalpy at each state
Our values are given as,
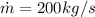
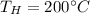
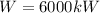
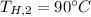
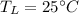
From the tables of Enthalpy of Water at 200°C (Saturated liquid state)
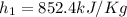
At the same time for 80°C
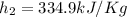
Applying the equation of Heat,
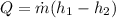
Replacing,
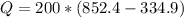
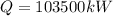
Therefore the efficiency would be
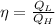
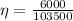
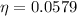
Therefore the actual thermal efficiency of the turbine in percent is 0.0579.