Answer:
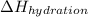 >
>
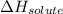 .
.
Step-by-step explanation:
Lattice enthalpy is defined as heat energy required to break 1 mole of crystal lattice. It is represented as
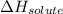 .
.
Hydration enthalpy is defined as amount energy released when 1 mole of ions undergo hydration (surrounding of water molecules).It is always negative. It is represented as
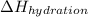 .
.
Enthalpy of solution = Lattice enthalpy + hydration enthalpy
If amplitude of lattice enthalpy > hydration enthalpy , enthalpy of solution will positive.Hence, solution will feel cool.
If magnitude Lattice enthalpy < hydration enthalpy, enthalpy of solution will negative. Hence solution will feel warm or hot.
Reaction between sodium hydroxide and water is an example of an exothermic reaction. During this process sodium hydroxide dissociates into sodium ions and hydroxide ions into the water.
Thus lattice enthalpy is lesser than the hydration enthalpy.