Answer:
Final temperature,
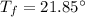
Step-by-step explanation:
Given that,
Mass of silver ring, m = 4 g
Initial temperature,
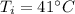
Heat released, Q = -18 J (as heat is released)
Specific heat capacity of silver,
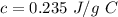
To find,
Final temperature
Solution,
The expression for the specific heat is given by :
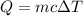

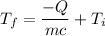
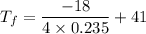
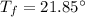
So, the final temperature of silver is 21.85 degrees Celsius.